
Should Bone Turnover Markers be routinely monitored when discontinuing someone from denosumab?

Should Bone Turnover Markers be routinely monitored when discontinuing someone from denosumab?
Contributors: Dr. Adrian Lau, Dr. Claudia Gagnon, Dr. Laëtitia Michou, Dr. Emma Billington, Dr. Zahra Bardai, Dr. Dr. Vithika Sivabalasundaram, Dr. Rowena Ridout, Dr. Sandra Kim, Dr. Suzanne Morin
Recommendations from Osteoporosis Canada Rapid Response Team.
Discontinuation of denosumab therapy is associated with rapid decline of bone mineral density, increase of bone turnover, and may result in multiple vertebral fractures (1). Osteoporosis Canada previously published a position statement about this (2).
Hence, the 2023 Osteoporosis Canada guideline (3) has recommended that those who are on denosumab therapy should remain on it long-term and ensure that therapy is uninterrupted.

The optimal duration of denosumab therapy is debatable. Studies have shown effectiveness of denosumab therapy with regards to improvements of BMD and fracture reduction, for up to 10 years (4). Beyond 10 years, there is a paucity of data.
Similar to bisphosphonates, long-term use of denosumab has been shown to be associated with osteonecrosis of the jaw (ONJ) and atypical femur fractures (AFFs). As the incidence of AFF and ONJ are very low, it is difficult to determine whether the use of denosumab results in higher risks of AFF and ONJ (5).
Given the BMD decline and increased risk of multiple vertebral compression fractures with the discontinuation of denosumab, routine discontinuation of denosumab or taking a drug holiday after a predetermined duration is not recommended.
However, there may be situations when denosumab therapy needs to be discontinued. These may include intolerance or side effects to denosumab, patient preference, or a choice to stop therapy after long term therapy, given the fear of the risk of ONJ and AFF.
There have been many studies assessing the optimal method of discontinuing denosumab. After a review of the literature, the authors of the 2023 Osteoporosis Canada guideline recommended the following:

In line with the European Calcified Society (ECTC)’s statement (6), Osteoporosis Canada recommends that for those who have had 5 or more doses of denosumab (i.e. 2.5 years or more of treatment), the advice from a consultant with expertise in osteoporosis should be sought on how to transition to an alternative therapy.
There are reasons why Osteoporosis Canada did not provide specific recommendations for discontinuation of denosumab for these patients.
- The 2023 guideline is aimed for primary care physicians as opposed to specialists.
- Transition will likely involve IV bisphosphonate therapy, which may need to be arranged at a hospital or an infusion centre.
- Not all provincial/territorial drug coverage plans provide reimbursement for more than one dose of IV bisphosphonate annually.
- There is ongoing research in this field, and the best way for transitioning someone off denosumab is not currently known and may also change with time.
The ECTS recommends the following:
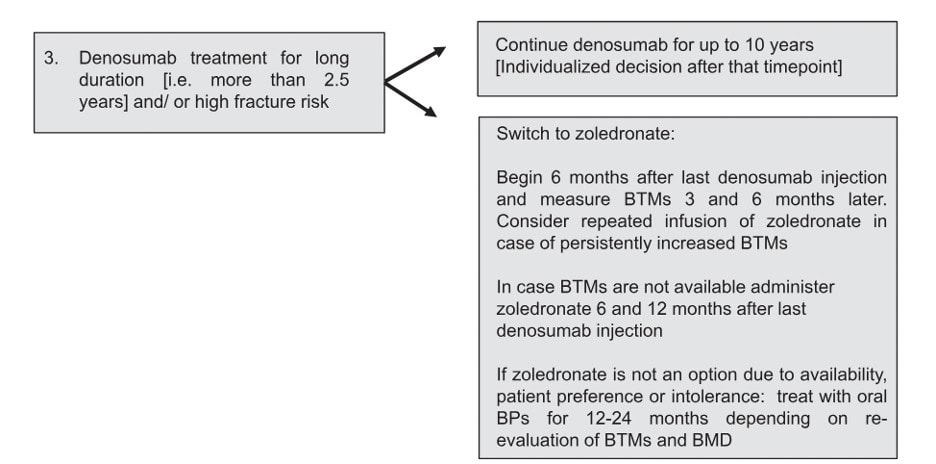
The Endocrine Society suggests that IV zoledronic acid be given at 8 months after the last dose of denosumab, and that the use of bone turnover markers may help determine when/if a second dose of zoledronic acid may be necessary (7).
A bone turnover marker target of C-terminal telopeptide (CTX) < 280 ng/L has been proposed as a target for adequate response after bisphosphonate therapy, by both the ECTS and Endocrine Society statements above. However, newer studies suggest that a lower target of CTX at 212 ng/L may be better at predicting possible bone loss (8). This shows that guidance on this matter will continue to evolve as new evidence continues to emerge.
The 2023 Osteoporosis Canada guideline has not made reference to or endorsed the use of bone turnover markers in the context of denosumab discontinuation. Although bone turnover markers may be available in most of Canada, it may not be universally funded, and hence, accessibility may be an issue. Although the lack of suppression after a dose of IV zoledronic acid may be an indication for the need of another dose, assessing the change in BMD may be a good alternative. The Korean Endocrine Society completed a review on this topic in 2022 (9). They recommend monitoring serum CTX and BMD, but that the decision to redose can be decided if the CTX is persistently elevated or if the BMD shows a significant decline.

The above recommendations from the various societies (ECTS, Endocrine Society, Korean Endocrine Society), are mostly based on expert opinion, rather than on established evidence. The divergence amongst the different recommendations reflects the differences in opinions amongst different experts.
Given that monitoring of BMD changes is an alternative to following bone turnover markers, and that not all Canadians have equal access to this test, we recommend that the decision to use bone turnover markers be made jointly by the patient and their physician.
Factors that may help with this decision include:
- Accessibility and coverage of the test
- Accessibility of IV zoledronic acid, including coverage for multiple doses within one year, if deemed necessary
- Other clinical evidence of bone stability (stability of BMD, absence or presence of new fractures)
- New evidence from research for or against the routine use of bone turnover markers for denosumab discontinuation
- Experience of the physician with interpretation of bone turnover marker results (aware of appropriate timing of test, fasting state, and consideration of factors influencing results).
References:
- Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018 Apr 23;190(16):E485-E486. doi: 10.1503/cmaj.180115.
- https://osteoporosis.ca/position-statements/increased-risk-of-vertebral-fracture-after-stopping-denosumab/
- Morin SN, Feldman S, Funnell L, Giangregorio L, Kim S, McDonald-Blumer H, Santesso N, Ridout R, Ward W, Ashe MC, Bardai Z, Bartley J, Binkley N, Burrell S, Butt D, Cadarette SM, Cheung AM, Chilibeck P, Dunn S, Falk J, Frame H, Gittings W, Hayes K, Holmes C, Ioannidis G, Jaglal SB, Josse R, Khan AA, McIntyre V, Nash L, Negm A, Papaioannou A, Ponzano M, Rodrigues IB, Thabane L, Thomas CA, Tile L, Wark JD; Osteoporosis Canada 2023 Guideline Update Group. Clinical practice guideline for management of osteoporosis and fracture prevention in Canada: 2023 update. CMAJ. 2023 Oct 10;195(39):E1333-E1348. doi: 10.1503/cmaj.221647.
- Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017 Jul;5(7):513-523. doi: 10.1016/S2213-8587(17)30138-9.
- Everts-Graber J, Bonel H, Lehmann D, Gahl B, Häuselmann H, Studer U, Ziswiler HR, Reichenbach S, Lehmann T. Incidence of Atypical Femoral Fractures in Patients on Osteoporosis Therapy-A Registry-Based Cohort Study. JBMR Plus. 2022 Sep 22;6(10):e10681. doi: 10.1002/jbm4.10681.
- Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2020 Oct 26:dgaa756. doi: 10.1210/clinem/dgaa756.
- Eastell R, Rosen CJ. Response to Letter to the Editor: “Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline”. J Clin Endocrinol Metab. 2019 Aug 1;104(8):3537-3538. doi: 10.1210/jc.2019-00777.
- Grassi G, Ghielmetti A, Zampogna M, Chiodini I, Arosio M, Mantovani G, Eller Vainicher C. Zoledronate after denosumab discontinuation: Is repeated administrations more effective than single infusion? J Clin Endocrinol Metab. 2024 Apr 13:dgae224. doi: 10.1210/clinem/dgae224.
- Tay WL, Tay D. Discontinuing Denosumab: Can It Be Done Safely? A Review of the Literature. Endocrinol Metab (Seoul). 2022 Apr;37(2):183-194. doi: 10.3803/EnM.2021.1369.
Scientific Advisory Council
Osteoporosis Canada’s rapid response team, made up of members of the Scientific Advisory Council, creates position statements as news breaks regarding osteoporosis. The position statements are used to inform both the healthcare professional and the patient. The Scientific Advisory Council (SAC) is made up of experts in Osteoporosis and bone metabolism and is a volunteer membership.

